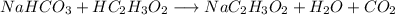Chemistry, 24.04.2020 01:54 brionesoswaldo09
You want to help your little brother make an exploding volcano for his science class. The lava will be made from reacting baking soda (NaHCO3) with vinegar (HC2H3O2). After building the volcano, you know that you want to create about 100.0g of lava (or sodium acetate, NaC2H3O2). Too little lava, and the volcano won’t overflow. Too much lava would be a giant mess! Using stoichiometry and the equation below, calculate the exact amount of baking soda needed to make 100.0g of lava. Assume you have excess vinegar. NaHCO3 + HC2H3O2 → NaC2H3O2 + H2O + CO2

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
You want to help your little brother make an exploding volcano for his science class. The lava will...
Questions

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20



Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Geography, 17.02.2021 04:20

Biology, 17.02.2021 04:20


History, 17.02.2021 04:20


Biology, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20



Biology, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20

Mathematics, 17.02.2021 04:20