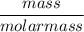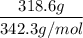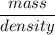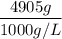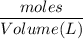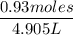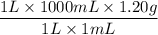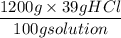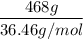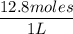A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the molarity of the solution
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the molarity of the HCl, if the solution has a density of 1.20 g/mL.
Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the mol of the HCl, if the solution has a density of 1.20 g/mL.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the m...
Questions

Mathematics, 28.01.2020 22:01



Social Studies, 28.01.2020 22:01

History, 28.01.2020 22:01

Mathematics, 28.01.2020 22:01



English, 28.01.2020 22:01


Biology, 28.01.2020 22:01

Biology, 28.01.2020 22:01

Geography, 28.01.2020 22:01

History, 28.01.2020 22:01