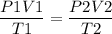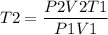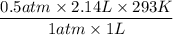Chemistry, 24.04.2020 00:35 austintules2005
The initial temperature of a 1.00 liter sample of argon is 293 K. The pressure is decreased from
720 mm Hg to 360 mm Hg and the volume increases to 2.14 liters. What was the final
temperature of the argon in Kelvin?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
The initial temperature of a 1.00 liter sample of argon is 293 K. The pressure is decreased from
Questions





Mathematics, 10.04.2020 19:01


Biology, 10.04.2020 19:01

Mathematics, 10.04.2020 19:01

Mathematics, 10.04.2020 19:01

Mathematics, 10.04.2020 19:01


Mathematics, 10.04.2020 19:01





English, 10.04.2020 19:01