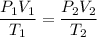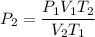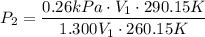Chemistry, 23.04.2020 22:23 christiantorres57
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of -161. °C. Suppose the temperature of a sample of methane gas is raised from -13.0 °C to 17.0°C, and at the same time the pressure is changed. If the initial pressure was 0.26 kPa and the volume increased by 30.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions

Geography, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Chemistry, 06.11.2020 01:00


Spanish, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

History, 06.11.2020 01:00

Chemistry, 06.11.2020 01:00



Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


English, 06.11.2020 01:00

English, 06.11.2020 01:00