
Chemistry, 23.04.2020 21:31 janighad01
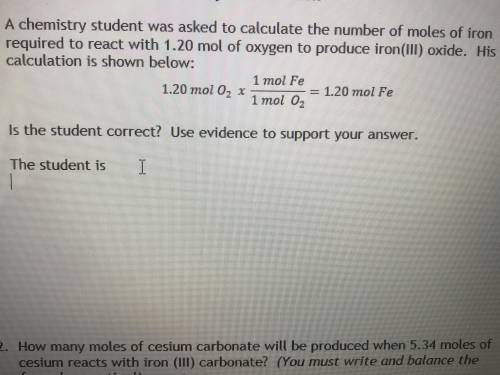
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 mol of oxygen to produce iron (iii) oxide. his calculation is shown below :
1.20 mol O2 x 1 mol fe / 1 mol O2 = 1.20 mol Fe
Is the student correct? Use evidence to support your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
A chemistry student was asked to calculate the number of moles of iron required to react with 1.20 m...
Questions

Health, 03.03.2021 01:00


Social Studies, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00


History, 03.03.2021 01:00

Social Studies, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

Social Studies, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Computers and Technology, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00




