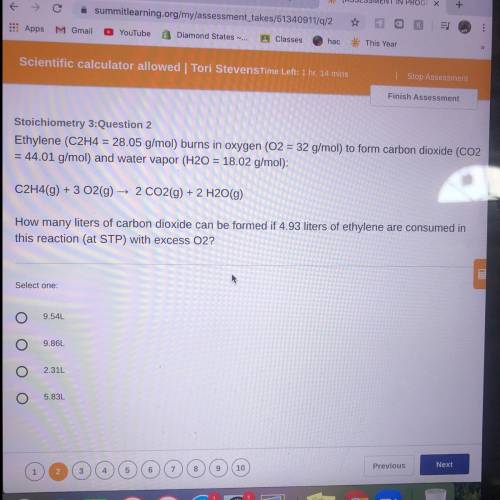
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44...

Chemistry, 23.04.2020 20:23 tchase0616
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44.01 g/mol) and water vapor (H20 = 18.02 g/mol):
C2H4(9) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
How many liters of carbon dioxide can be formed if 4.93 liters of ethylene are consumed in
this reaction (at STP) with excess O2?
Select one:
9.54L
9.86L
2.31L
5.83L

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2


Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
Questions




Computers and Technology, 01.08.2019 02:00



Physics, 01.08.2019 02:00

Advanced Placement (AP), 01.08.2019 02:00


Spanish, 01.08.2019 02:00


History, 01.08.2019 02:00

Mathematics, 01.08.2019 02:00



Business, 01.08.2019 02:00

Social Studies, 01.08.2019 02:00

Social Studies, 01.08.2019 02:00




