Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1


Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
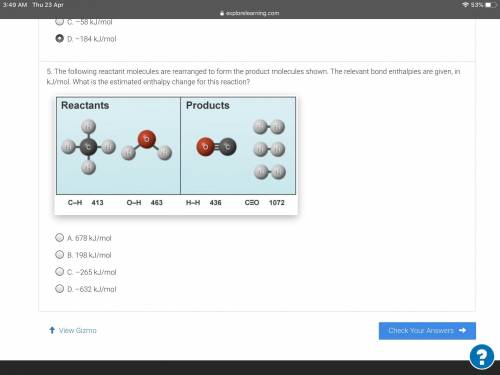
The following reactant molecules are rearranged to form the product molecules shown. The relevant bo...
Questions

Chemistry, 30.01.2020 19:45










History, 30.01.2020 19:45


Physics, 30.01.2020 19:45

Mathematics, 30.01.2020 19:45


History, 30.01.2020 19:45

English, 30.01.2020 19:45


Mathematics, 30.01.2020 19:45