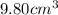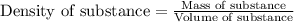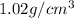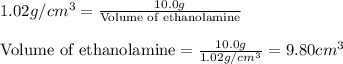Chemistry, 23.04.2020 02:10 abigailweeks10
A chemistry student needs 10.0g of ethanolamine for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of ethanolamine is ·1.02gcm−3. Calculate the volume of ethanolamine the student should pour out.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

You know the right answer?
A chemistry student needs 10.0g of ethanolamine for an experiment. By consulting the CRC Handbook of...
Questions



History, 28.07.2019 17:30


Biology, 28.07.2019 17:30

Mathematics, 28.07.2019 17:30

Social Studies, 28.07.2019 17:30

Biology, 28.07.2019 17:30

History, 28.07.2019 17:30

Mathematics, 28.07.2019 17:30

Social Studies, 28.07.2019 17:30

Social Studies, 28.07.2019 17:30




Social Studies, 28.07.2019 17:30


Mathematics, 28.07.2019 17:30