

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
The Ksp of Al(OH)3 at 25 oC is 1 x 10-33. Consider a solution that is 1.0 x 10-10 M Al(NO3)3 and 1.0...
Questions

Mathematics, 14.04.2021 16:00


Arts, 14.04.2021 16:00


English, 14.04.2021 16:00





Social Studies, 14.04.2021 16:00


Computers and Technology, 14.04.2021 16:00

Mathematics, 14.04.2021 16:00

Mathematics, 14.04.2021 16:00






Health, 14.04.2021 16:00

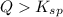 and a precipitate will form.
and a precipitate will form.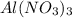 =
= 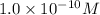
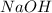 =
= 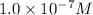
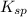 of
of 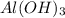 =
= 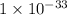
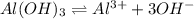
![Q=[Al^{3+}][OH^-]^3](/tpl/images/0619/9788/355c8.png)
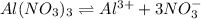
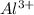 =
= 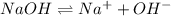
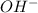 =
= 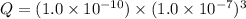
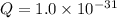
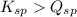 ; the reaction is product favored. (No precipitation)
; the reaction is product favored. (No precipitation)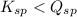 ; the reaction is reactant favored. (Precipitation)
; the reaction is reactant favored. (Precipitation)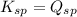 ; the reaction is in equilibrium. (Sparingly soluble)
; the reaction is in equilibrium. (Sparingly soluble)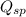 is more than
is more than 

