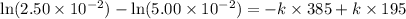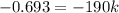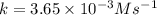Chemistry, 22.04.2020 22:57 grayjasmine46
The reactant concentration in a zero-order reaction was 5.00×10−2 M after 195 s and 2.50×10−2 M after 385 s . What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
The reactant concentration in a zero-order reaction was 5.00×10−2 M after 195 s and 2.50×10−2 M afte...
Questions

Advanced Placement (AP), 06.11.2019 03:31



Health, 06.11.2019 03:31


Mathematics, 06.11.2019 03:31



Mathematics, 06.11.2019 03:31

Physics, 06.11.2019 03:31




Advanced Placement (AP), 06.11.2019 03:31


Mathematics, 06.11.2019 03:31


Mathematics, 06.11.2019 03:31

Mathematics, 06.11.2019 03:31

Mathematics, 06.11.2019 03:31

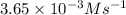
![\ln [A]=-kt+\ln [A_o]](/tpl/images/0619/6365/bdc3f.png)
![[A_o]](/tpl/images/0619/6365/dc622.png) = initial concentration
= initial concentration![[A]](/tpl/images/0619/6365/6aa06.png) = final concentration =
= final concentration = 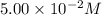 at 195 s
at 195 s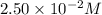 at 385 s
at 385 s![\ln (5.00\times 10^{-2})=-k\times 195+\ln [A_o]](/tpl/images/0619/6365/3a318.png) ............(1)
............(1)![\ln (2.50\times 10^{-2})=-k\times 385+\ln [A_o]](/tpl/images/0619/6365/497e4.png) ............(2)
............(2)![\ln (2.50\times 10^{-2})-\ln (5.00\times 10^{-2})=-k\times 385+\ln [A_o]+k\times 195-\ln [A_o]](/tpl/images/0619/6365/4b64f.png)