

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
The true absorbance for a 1.0 x 10 −5 M solution is 0.7526. If the percentage stray light for a spec...
Questions



Biology, 21.08.2019 00:00

History, 21.08.2019 00:00

English, 21.08.2019 00:00

Social Studies, 21.08.2019 00:00


Mathematics, 21.08.2019 00:00

Mathematics, 21.08.2019 00:00



Mathematics, 21.08.2019 00:00

Biology, 21.08.2019 00:00

Mathematics, 21.08.2019 00:00

Biology, 21.08.2019 00:00

Geography, 21.08.2019 00:00

History, 21.08.2019 00:00


Mathematics, 21.08.2019 00:00

Chemistry, 21.08.2019 00:00

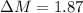 %
%
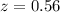 %
% 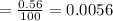
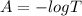
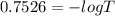
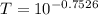
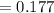
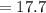 %
%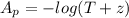
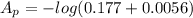
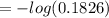
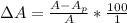
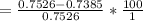
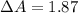 %
% 

