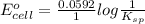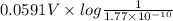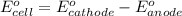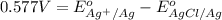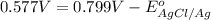Chemistry, 22.04.2020 22:03 Buttercream16
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the solubility product constant for AgCl at 298 K is 1.77 × 10 − 10 and the standard reduction potential of the half‑reaction Ag + ( aq ) + e − − ⇀ ↽ − Ag ( s ) is + 0.799 V .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the...
Questions

Computers and Technology, 20.01.2022 14:00

Computers and Technology, 20.01.2022 14:00


English, 20.01.2022 14:00


Mathematics, 20.01.2022 14:00

English, 20.01.2022 14:00



Mathematics, 20.01.2022 14:00


Mathematics, 20.01.2022 14:00

Computers and Technology, 20.01.2022 14:00

Geography, 20.01.2022 14:00

Mathematics, 20.01.2022 14:00

Mathematics, 20.01.2022 14:00

Computers and Technology, 20.01.2022 14:00


Social Studies, 20.01.2022 14:00

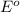 for the half-cell reaction is 0.222 V.
for the half-cell reaction is 0.222 V.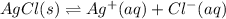
![K_{sp} = [Ag^{+}][Cl^{-}]](/tpl/images/0619/3632/031c0.png)
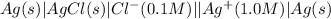
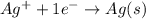 ,
, 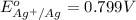
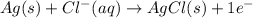 ,
, 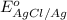 = ?
= ?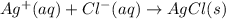
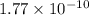
![E_{cell} = E^{o}_{cell} - \frac{0.0592 V}{n} log \frac{[AgCl]}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/d09c5.png)
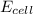 = 0.00 V
= 0.00 V![0.00 = E^{o}_{cell} - \frac{0.0592 V}{1} log \frac{1}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/1250d.png)