Chemistry, 22.04.2020 21:55 felipeee4609
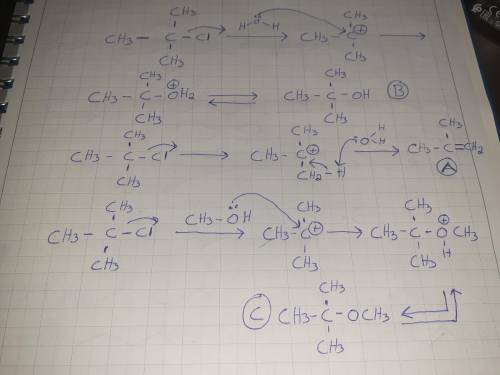
Treating (CH3)3C-Cl with a mixture of H2O and CH3OH at room temperature would yield: A) CH2=C(CH3)2 B) (CH3)3COH C) (CH3)3COCH3 D) All of these choices. E) None of these choices.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
Treating (CH3)3C-Cl with a mixture of H2O and CH3OH at room temperature would yield: A) CH2=C(CH3)2...
Questions


Geography, 09.03.2021 20:40


Mathematics, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40


Mathematics, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40


German, 09.03.2021 20:40



Mathematics, 09.03.2021 20:40

Mathematics, 09.03.2021 20:40


Mathematics, 09.03.2021 20:40

English, 09.03.2021 20:40

History, 09.03.2021 20:40

History, 09.03.2021 20:40