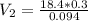Chemistry, 22.04.2020 19:52 blink182lovgabbie
During your reaction, you added 0.3 mL concentrated H2SO4 (18.4 M) as the catalyst. At the end of the reaction, you need to add base to neutralize it. How much volume (in mL) of 10% Na2CO3 to neutralize all the acid present

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
During your reaction, you added 0.3 mL concentrated H2SO4 (18.4 M) as the catalyst. At the end of th...
Questions


Mathematics, 18.12.2020 04:50

English, 18.12.2020 04:50

Health, 18.12.2020 04:50

History, 18.12.2020 04:50

Mathematics, 18.12.2020 04:50

Mathematics, 18.12.2020 04:50

Mathematics, 18.12.2020 04:50



History, 18.12.2020 04:50


Mathematics, 18.12.2020 04:50


SAT, 18.12.2020 04:50



History, 18.12.2020 04:50


Mathematics, 18.12.2020 04:50