
Chemistry, 22.04.2020 19:00 arieannaensley0616
Given the balanced equation 2C4H10 + 13O2 → 8CO2 + 10H2O, how many moles of CO2 are produced when 14.9g of O2 are used?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Given the balanced equation 2C4H10 + 13O2 → 8CO2 + 10H2O, how many moles of CO2 are produced when 14...
Questions


Chemistry, 06.01.2021 19:40






History, 06.01.2021 19:40

Mathematics, 06.01.2021 19:40


Mathematics, 06.01.2021 19:40





Biology, 06.01.2021 19:40


Mathematics, 06.01.2021 19:40


Mathematics, 06.01.2021 19:40

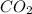 produced are, 0.287 moles.
produced are, 0.287 moles.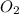 = 14.9 g
= 14.9 g
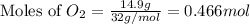
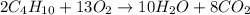
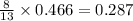 mole of
mole of 

