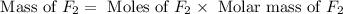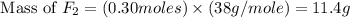Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Phosphorus trifluoride is formed from its elements P4 (s) F2 (g) ---> PF3 (g) how many grams of f...
Questions





Engineering, 18.03.2021 01:20


Social Studies, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20







Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Computers and Technology, 18.03.2021 01:20

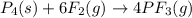
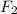 needed are, 11.4 grams.
needed are, 11.4 grams.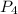 = 6.20 g
= 6.20 g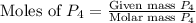
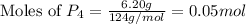
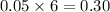 moles of
moles of