Fe
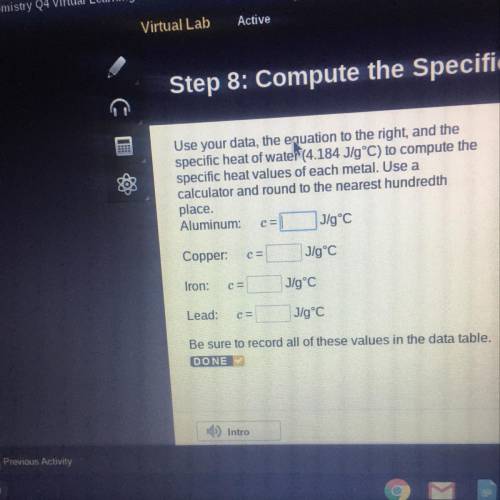
Use your data, the equation to the night, and the
specific heat of water14.184 Jig')...

Chemistry, 22.04.2020 07:47 cindyc1103
Fe
Use your data, the equation to the night, and the
specific heat of water14.184 Jig') to compute the
Specific heat values of each metal, Use a
calculator and round to the nearest hundredth
place
Aluminuntelec
Mynter (g)
39.85
40 13
4024
39 66
Mmetai (g)
11.98
12.14
12 31
1246
Copper
tron:
JgC
319°C
Arumer (°C)
47 19 24
| 129 | 154 | 151
07
167
ATmes (°C)
Load:
Jlg'
Be sure to record all of these values in the data table
Cre
a te Mater AT
Metsretal
Intro

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Microtubular fibers that assist in the movement of chromosomes during nuclear division in conjunction with proteins, makes up the small and large organelle pieces that assemble prior to translation three-base nucleotide sequence that can complementarily pair with the functional transcript, allowing a particular material to be brought to a ribosome particular time in the cell cycle when the cell’s systems determine if the cellular conditions are appropriate to continue through the cycle time in the cell’s cycle when proteins are made and organelles are duplicated enzyme that allows proper nucleotide bonding during transcription specific dna sequence which will initiate gene transcription division of the cell’s cytoplasm specific bond that forms between two amino acids when a carboxyl group binds to a amino group three-base sequence that does not code for a particular amino acid a paired organelle which facilitates the formation of movement microtubules time in the cell’s cycle when the microtubular structures exert an equal pressure on the cell’s genetic material
Answers: 2


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 23.06.2019 21:00
Sulfuryl dichloride may be formed from the reaction of sulfur dioxide and chlorine. so2(g) + cl2(g) → so2cl2(g) substance: so2(g) cl2(g) so2cl2(g) δh°f (kj/mol) at 298 k –296.8 0 –364.0 δg°f (kj/mol) at 298 k –300.1 0 –320.0 s°(j/k • mol) at 298 k 248.2 223.0 311.9 what is δg°rxn for this reaction at 600 k?
Answers: 2
You know the right answer?
Questions



Mathematics, 03.04.2020 18:01

Geography, 03.04.2020 18:01








Biology, 03.04.2020 18:02











