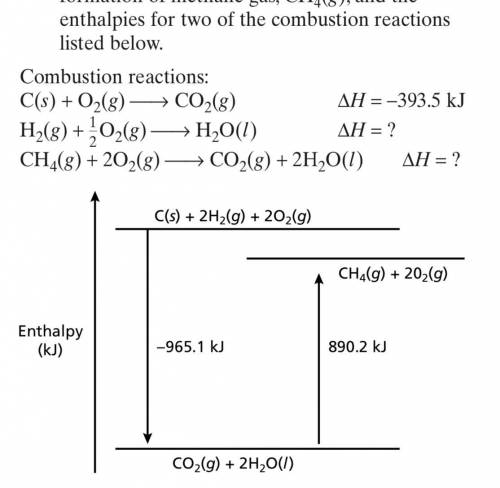
For certain molecules, enthalpies of formation
can be determined from combustion data. Using t...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Questions



Mathematics, 09.01.2021 01:40

Mathematics, 09.01.2021 01:40



History, 09.01.2021 01:40

Mathematics, 09.01.2021 01:40

Business, 09.01.2021 01:40


Mathematics, 09.01.2021 01:40


Chemistry, 09.01.2021 01:40




Chemistry, 09.01.2021 01:40

Mathematics, 09.01.2021 01:40