Chemistry, 22.04.2020 04:59 jwagner1580
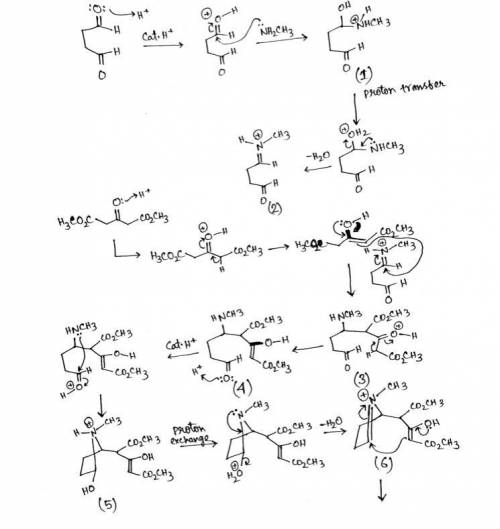
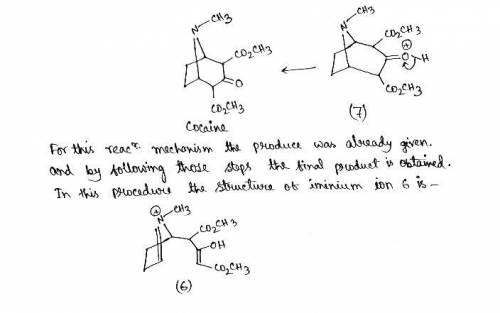
Cocaine has been prepared by a sequence beginning with a Mannich reaction between dimethyl acetonedicarboxylate, methylamine, and butanedial. The mechanism involves the following steps: 1. Following initial protonation of the carbonyl oxygen, nucleophilic attack by the amine forms carbinolamine 1; 2. Proton transfer and elimination of water forms iminium ion 2; 3. The enol form of the dicarboxylate ester attacks the iminium ion to form adduct 3; 4. Adduct 3 tautomerizes to form enol 4; 5. Following protonation of the aldehyde, cyclization occurs as protonated carbinolamine 5 is formed; 6. Proton transfer and elimination of water lead to iminium ion 6; 7. The enol attacks the iminium ion in a second cyclization reaction to form bicyclic ion 7; 8. Deprotonation leads to the final product. Write out the mechanism on a separate sheet of paper and then draw the structure of protonated carbinolamine 1.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2


You know the right answer?
Cocaine has been prepared by a sequence beginning with a Mannich reaction between dimethyl acetonedi...
Questions

Mathematics, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00


Chemistry, 09.12.2020 20:00


Social Studies, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00

Arts, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00


Geography, 09.12.2020 20:00

English, 09.12.2020 20:00