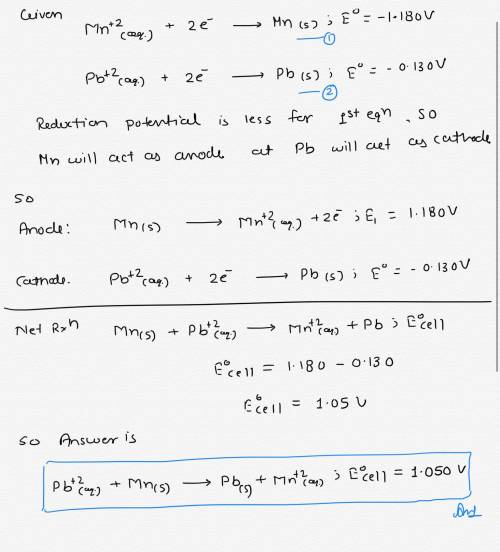
Which of the following is the balanced overall reaction and standard cell potential of an electrochemical cell constructed from half-cells with the given half reactions? Mn 2+(aq) + 2 e−→Mn(s); E° = –1.180 V Pb2+(aq) + 2 e−→ Pb(s); E° = –0.130 V Group of answer choices Pb 2+(aq) + Mn(s) →Pb(s) + Mn2+(aq); = 1.050 V Pb(s) + Mn2+(aq) →Pb2+(aq) + Mn(s); = −1.050 V Pb 2+(aq) + Mn2+(aq) →Pb(s) + Mn(s); = –1.310 V Pb 2+(aq) + Mn(s) →Pb(s) + Mn2+(aq); =0.525 V Pb(s) + Mn2+(aq) →Pb2+(aq) + Mn(s); = −0.525 V

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3
You know the right answer?
Which of the following is the balanced overall reaction and standard cell potential of an electroche...
Questions

Mathematics, 17.11.2020 02:10




Chemistry, 17.11.2020 02:10

English, 17.11.2020 02:10

Social Studies, 17.11.2020 02:10



English, 17.11.2020 02:10

Spanish, 17.11.2020 02:10

English, 17.11.2020 02:10

Mathematics, 17.11.2020 02:10

History, 17.11.2020 02:10

History, 17.11.2020 02:10

Mathematics, 17.11.2020 02:10

Biology, 17.11.2020 02:10

Mathematics, 17.11.2020 02:10

Mathematics, 17.11.2020 02:10

Biology, 17.11.2020 02:10