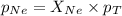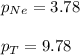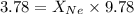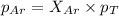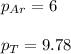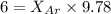Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
A vessel containing Ne(g) and Ar(g) has a total pressure of 9.78. If the partial pressure of the Neo...
Questions

Business, 16.07.2020 08:01

History, 16.07.2020 08:01

Mathematics, 16.07.2020 08:01

Social Studies, 16.07.2020 08:01

Mathematics, 16.07.2020 08:01


English, 16.07.2020 08:01




Computers and Technology, 16.07.2020 08:01



Geography, 16.07.2020 08:01



Mathematics, 16.07.2020 08:01

Spanish, 16.07.2020 08:01

Spanish, 16.07.2020 08:01

Biology, 16.07.2020 08:01

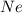 and
and 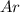 is, 0.387 and 0.613 respectively.
is, 0.387 and 0.613 respectively.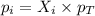
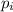 = partial pressure of gas
= partial pressure of gas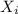 = mole fraction of gas
= mole fraction of gas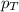 = total pressure of gas
= total pressure of gas