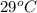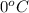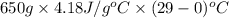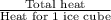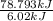Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume that each ice cube contains 1 mole of H2O and is initially at 0°C. ∆H(fusion) = 6.02 kJ/mol; ∆H(vaporization) = 40.7 kJ/mol c(solid) = 2.09 J/g°C; c(liquid) = 4.18 J/g°C; c(gas) = 1.97 J/g°C Enter your answer numerically.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume...
Questions

Biology, 03.12.2021 04:20


Health, 03.12.2021 04:20

Mathematics, 03.12.2021 04:20

History, 03.12.2021 04:20



History, 03.12.2021 04:20

Computers and Technology, 03.12.2021 04:20

Mathematics, 03.12.2021 04:20





Advanced Placement (AP), 03.12.2021 04:20

Biology, 03.12.2021 04:20


English, 03.12.2021 04:20


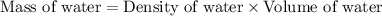
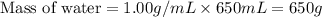
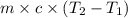
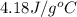
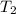 = final temperature =
= final temperature =