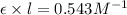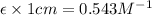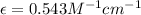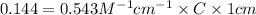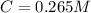Chemistry, 22.04.2020 03:34 fruitasticgabs8175
After creating a Beer's Law plot using standard solutions of Q, you determined the slope of Beer's Law to be 0.543 M-1. Your unknown solution of Q tested in Part B of the experiment had an absorbance of 0.144. Determine the concentration (in molarity) of the unknown solution Q from Part B. Do not use scientific notation or units in your response. If Carmen adds zeros after the decimal place, your answer will still be graded correctly.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
After creating a Beer's Law plot using standard solutions of Q, you determined the slope of Beer's L...
Questions


Health, 14.01.2020 01:31

Mathematics, 14.01.2020 01:31


Mathematics, 14.01.2020 01:31

Physics, 14.01.2020 01:31


Mathematics, 14.01.2020 01:31


Chemistry, 14.01.2020 01:31

Health, 14.01.2020 01:31


Biology, 14.01.2020 01:31

English, 14.01.2020 01:31

History, 14.01.2020 01:31

Mathematics, 14.01.2020 01:31


Mathematics, 14.01.2020 01:31

Geography, 14.01.2020 01:31

Mathematics, 14.01.2020 01:31


 = molar absorptivity coefficient
= molar absorptivity coefficient and path length is 1 cm.
and path length is 1 cm.