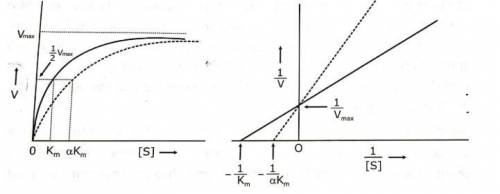
In cases of ethylene glycol poisoning, treatment involves administration of Ethanol (grain alcohol), which works by competitively inhibiting ADH, an enzyme that oxidizes ethylene glycol to organic acids. As a competitive inhibitor, ethanol: decreases apparent Km without affecting Vmax· increases apparent Vmax without affecting Km. decreases both apparent Vmax and apparent Km. increases apparent Km without affecting Vmax· decreases apparent Vmax without affecting Km.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
In cases of ethylene glycol poisoning, treatment involves administration of Ethanol (grain alcohol),...
Questions



Computers and Technology, 12.08.2020 05:01




Mathematics, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01