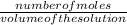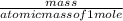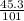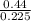*multiple choice*
What is the molarity of a 45.3g sample of KNO3 (101g) dissolved in enough w...

Chemistry, 22.04.2020 02:26 katlynnschmolke
*multiple choice*
What is the molarity of a 45.3g sample of KNO3 (101g) dissolved in enough water to make a 0.225L solution?
a) 0.45
b) 2
c) option 3
d) none of the above

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Questions







Biology, 12.02.2021 21:00





Mathematics, 12.02.2021 21:00


Mathematics, 12.02.2021 21:00

Spanish, 12.02.2021 21:00


Physics, 12.02.2021 21:00

Mathematics, 12.02.2021 21:00

Mathematics, 12.02.2021 21:00

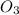 = 45.3 grams
= 45.3 grams