
Chemistry, 22.04.2020 01:56 julielebo8
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction system. b. the increased molecular disorder that occurs in the system. c. the energy given off to the surroundings. d. the direction in which a net reaction occurs. e. the excess entropy given off to the surroundings

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction...
Questions


Computers and Technology, 01.10.2019 14:10

Mathematics, 01.10.2019 14:10


English, 01.10.2019 14:10



Biology, 01.10.2019 14:10



History, 01.10.2019 14:10


Mathematics, 01.10.2019 14:10


English, 01.10.2019 14:10



Computers and Technology, 01.10.2019 14:10

History, 01.10.2019 14:10

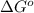 . Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.
. Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.

