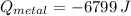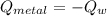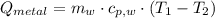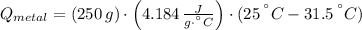Chemistry, 22.04.2020 02:39 rodolfoperezzz1332
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initially at 25.0 oC. The final temperature of the water is 31.5 oC. What is the heat change of the metal in joules? The specific heat of water is 4.184 J/goC

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

You know the right answer?
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initial...
Questions

Biology, 07.06.2021 16:40

Mathematics, 07.06.2021 16:40



Mathematics, 07.06.2021 16:40


Mathematics, 07.06.2021 16:40

Geography, 07.06.2021 16:40

Law, 07.06.2021 16:40



Biology, 07.06.2021 16:40

Mathematics, 07.06.2021 16:40


Mathematics, 07.06.2021 16:40

Mathematics, 07.06.2021 16:40

History, 07.06.2021 16:40

Mathematics, 07.06.2021 16:40