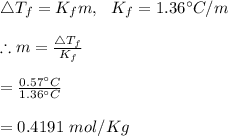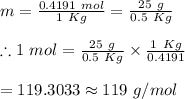Chemistry, 22.04.2020 01:46 slowik9467
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
0.57 °C what is the molecular weight of the compound assume no molecular disassociation upon
dissolution Kf equals 1.36 °C/m
O 119 g/mol
90 g/mol
0 60 g/mol
238 g/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

You know the right answer?
25 g of a compound is added to 500 mL of water if the freezing point of the resulting solution is
Questions

History, 06.10.2019 10:10

Mathematics, 06.10.2019 10:10

Mathematics, 06.10.2019 10:10

Spanish, 06.10.2019 10:10

Mathematics, 06.10.2019 10:10

Mathematics, 06.10.2019 10:10

Physics, 06.10.2019 10:10

Biology, 06.10.2019 10:10



Mathematics, 06.10.2019 10:10


Biology, 06.10.2019 10:10

Chemistry, 06.10.2019 10:10



Mathematics, 06.10.2019 10:10

Chemistry, 06.10.2019 10:10

Chemistry, 06.10.2019 10:10

Chemistry, 06.10.2019 10:20