
Chemistry, 22.04.2020 00:05 kailibug2287
Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white precipitate, presumably AgCl. You dissolve 0.1000 g of the complex in H2O and perform a precipitation titration with 0.0500 M AgNO3 as the titrant. Using an electrode that is sensitive to [Ag ], you reach the endpoint after 9.00 mL of titrant is added. How many grams of chloride ion were present in the 0.1000-g sample

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white precipitate, presuma...
Questions

Computers and Technology, 30.06.2019 03:30


Mathematics, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30


Mathematics, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30


English, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30




Mathematics, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30


Social Studies, 30.06.2019 03:30

Chemistry, 30.06.2019 03:30

Spanish, 30.06.2019 03:30

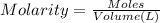
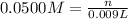
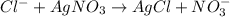
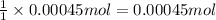 of chloride ions
of chloride ions

