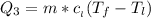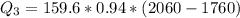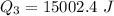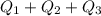Use the following information on Cr to determine the amount of heat required to convert 159.6 g of solid Cr at 1760°C into liquid Cr at 2060°C. melting point = 1860°C; boiling point = 2672°C ΔHfus = 20.5 kJ/mol; ΔHvap = 339 kJ/mol; c(solid) = 44.8 J/g°C; c(liquid) = 0.94 J/g°C Enter your answer in units of kJ to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Use the following information on Cr to determine the amount of heat required to convert 159.6 g of s...
Questions

Mathematics, 17.10.2019 03:00

Biology, 17.10.2019 03:00


Mathematics, 17.10.2019 03:00

Biology, 17.10.2019 03:00


Health, 17.10.2019 03:00

Computers and Technology, 17.10.2019 03:00

Biology, 17.10.2019 03:00



Biology, 17.10.2019 03:00

Mathematics, 17.10.2019 03:00





Social Studies, 17.10.2019 03:00


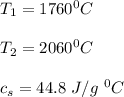
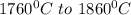 is calculated as:
is calculated as: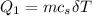
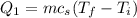

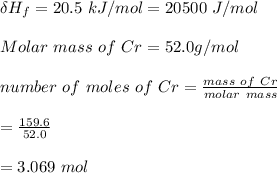
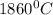 is;
is;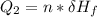
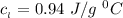
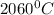 is;
is;