Chemistry, 21.04.2020 20:51 genyjoannerubiera
A gas in an engine cylinder expands from a volume of 10.0 L to 15.0 L against an external pressure of 1 atm and the system absorbs 300 J of heat in the process. Determine the work done by the system and the change in the system's internal energy, both in joules. Use this conversion scale to calculate the work done in joules: 1 L * atm

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
A gas in an engine cylinder expands from a volume of 10.0 L to 15.0 L against an external pressure o...
Questions

Mathematics, 17.08.2021 02:50

History, 17.08.2021 02:50


History, 17.08.2021 02:50

History, 17.08.2021 02:50

Physics, 17.08.2021 02:50





Mathematics, 17.08.2021 02:50

Mathematics, 17.08.2021 02:50



Mathematics, 17.08.2021 02:50

Mathematics, 17.08.2021 02:50



Mathematics, 17.08.2021 02:50

Mathematics, 17.08.2021 02:50

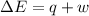
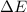 =Change in internal energy
=Change in internal energy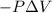 {Work is done by the system as the final volume is greater than initial volume and is negative}
{Work is done by the system as the final volume is greater than initial volume and is negative}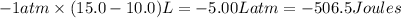 {1Latm=101.3J}
{1Latm=101.3J}