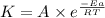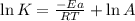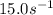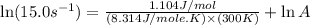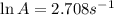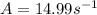A chemical reaction has an energy of activation Ea = 1∙104 J mol-1 at T = 300 K. The first-order rate constant for this reaction was found to be 15.0 s-1. In the presence of a catalyst, the activation energy is reduced to 1∙103 J mol-1. Calculate the pre-exponential factor in the Arrhenius equation

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1


Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
A chemical reaction has an energy of activation Ea = 1∙104 J mol-1 at T = 300 K. The first-order rat...
Questions


Biology, 24.11.2021 20:50

Mathematics, 24.11.2021 20:50


History, 24.11.2021 20:50






History, 24.11.2021 20:50


Arts, 24.11.2021 20:50



Mathematics, 24.11.2021 20:50

Mathematics, 24.11.2021 21:00



Mathematics, 24.11.2021 21:00