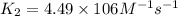Chemistry, 21.04.2020 18:50 xxaurorabluexx
For the second-order reaction NO( g) + O 3( g) → NO 2( g) + O 2( g), the rate constant has been measured to be 1.08 × 10 7 M –1 s –1 at 298 K and the activation energy has been measured to be 11.4 kJ/mol over the temperature range 195 K to 304 K. What is the rate constant at 207 K? ( R = 8.3145 J K –1 mol –1)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1


Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1
You know the right answer?
For the second-order reaction NO( g) + O 3( g) → NO 2( g) + O 2( g), the rate constant has been meas...
Questions


History, 20.09.2019 21:10










Mathematics, 20.09.2019 21:10








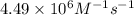
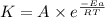
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0615/2426/6d953.png)
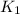 = rate constant at
= rate constant at 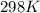 =
= 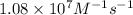
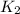 = rate constant at
= rate constant at 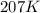 = ?
= ?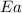 = activation energy for the reaction =
= activation energy for the reaction = 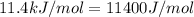
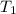 = initial temperature = 298 K
= initial temperature = 298 K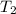 = final temperature = 207 K
= final temperature = 207 K![\log (\frac{K_2}{1.08\times 10^7M^{-1}s^{-1}})=\frac{11400J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{298K}-\frac{1}{207K}]](/tpl/images/0615/2426/e96a1.png)