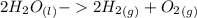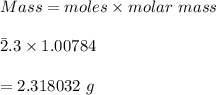Chemistry, 21.04.2020 18:47 PONBallfordM89
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseous elements. How many grams of hydrogen gas should get (theoretical yield)? The chemist collected 2.0 moles hydrogen gas. What is his percent yield?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1


You know the right answer?
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseo...
Questions

Mathematics, 12.01.2021 22:10


Health, 12.01.2021 22:10

Mathematics, 12.01.2021 22:10





Mathematics, 12.01.2021 22:10

Mathematics, 12.01.2021 22:10

Mathematics, 12.01.2021 22:10



English, 12.01.2021 22:10



Mathematics, 12.01.2021 22:10



Mathematics, 12.01.2021 22:10