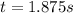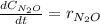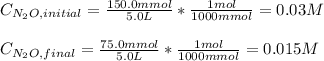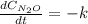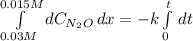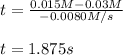Chemistry, 21.04.2020 17:30 amandajbrewerdavis
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of ·0.0080Ms−1: 2N2O(g)→2N2(g)+O2(g) Suppose a 5.0L flask is charged under these conditions with 150.mmol of dinitrogen monoxide. After how much time is there only 75.0mmol left? You may assume no other reaction is important.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1


You know the right answer?
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate...
Questions

Health, 16.10.2020 18:01

Social Studies, 16.10.2020 18:01



History, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01


History, 16.10.2020 18:01

History, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01




Mathematics, 16.10.2020 18:01


History, 16.10.2020 18:01

Computers and Technology, 16.10.2020 18:01

Advanced Placement (AP), 16.10.2020 18:01

History, 16.10.2020 18:01