
For the decomposition of phosphorous pentachloride to phosphorous trichloride and chlorine at 400K the KC is 1.1x10-2. Given that 1.0g of phosphorous pentachloride is added to a 250mL reaction flask, find the percent decomposition after the system has reached equilibrium. PCl_5(g) PCl_3(g) Cl_2(g) K_C

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1
You know the right answer?
For the decomposition of phosphorous pentachloride to phosphorous trichloride and chlorine at 400K t...
Questions




Mathematics, 11.02.2020 19:49

Chemistry, 11.02.2020 19:49


History, 11.02.2020 19:49









Chemistry, 11.02.2020 19:49




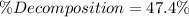
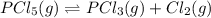
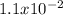 and the initial concentration of phosphorous pentachloride is:
and the initial concentration of phosphorous pentachloride is:![[PCl_5]_0=\frac{1.0gPCl_5*\frac{1molPCl_5}{208.24gPCl_5} }{250mL*\frac{1L}{1000mL} } =0.019M](/tpl/images/0614/9385/f2a4f.png)
![Kc=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0614/9385/c6686.png)
 occurring due to the reaction extent and the concentrations at equilibrium (ICE table methodology):
occurring due to the reaction extent and the concentrations at equilibrium (ICE table methodology):![Kc=\frac{(x)(x)}{[PCl_5]_0-x}=\frac{x^2}{0.019-x}=1.1x10^{-2}](/tpl/images/0614/9385/3162f.png)
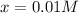
![[PCl_5]_{eq}=[PCl_5]_0-x=0.019M-0.01M\\](/tpl/images/0614/9385/8b1c4.png)
![[PCl_5]_{eq}=0.009M](/tpl/images/0614/9385/82584.png)
![\% Decomposition=\frac{[PCl_5]_0}{[PCl_5]_{eq}}*100\%=\frac{0.009M}{0.019M} *100\%\\\\\% Decomposition=47.4\%](/tpl/images/0614/9385/7bb6c.png)


