Chemistry, 21.04.2020 15:51 judyandaub1
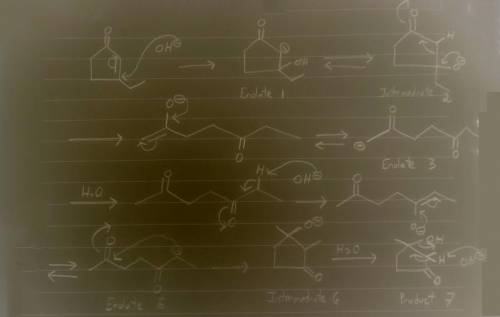
Aldol condensation of 2,5-heptanedione yields a mixture of two enone products in a 9:1 ratio. Treatment of the minor product with aqueous NaOH converts it into the major product; the interconversion proceeds as follows: Hydroxide ion adds to the double bond, forming enolate ion 1; Proton transfer occurs, yielding tetrahedral intermediate 2; Ring opening occurs, yielding enolate ion 3; Protonation of enolate ion 3 occurs, yielding 2,5-heptanedione; Deprotonation at C-6 occurs, yielding enolate ion 5; Enolate ion 5 attacks C-2, yielding tetrahedral intermediate 6; Protonation occurs to yield aldol addition product 7; Dehydration yields the more stable product.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Aldol condensation of 2,5-heptanedione yields a mixture of two enone products in a 9:1 ratio. Treatm...
Questions

History, 18.08.2020 23:01












Computers and Technology, 18.08.2020 23:01






Mathematics, 18.08.2020 23:01

History, 18.08.2020 23:01