
Chemistry, 21.04.2020 15:33 alexus6339
Consider a 125 mL buffer solution at 25°C that contains 0.500 mol of hypochlorous acid (HOCl) and 0.500 mol of sodium hypochlorite (NaOCl). What will be the pH of this buffer solution after adding 0.341 mol of HCl? The Ka of hypochlorous acid is 2.9 x 10-8 . Assume the change in volume of the buffer solution is negligible

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1


You know the right answer?
Consider a 125 mL buffer solution at 25°C that contains 0.500 mol of hypochlorous acid (HOCl) and 0....
Questions


Biology, 10.06.2020 23:57


Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57





Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57




French, 10.06.2020 23:57



Mathematics, 10.06.2020 23:57

Chemistry, 10.06.2020 23:57

![\frac{[NaOCl]}{[HOCl]}](/tpl/images/0614/6948/a0bd5.png)
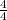 pH = 7.54
pH = 7.54![\frac{[NaOCl-HCl]}{[HOCl+HCl]}](/tpl/images/0614/6948/7bc1b.png) pH = 7.54 + log
pH = 7.54 + log 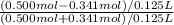 pH = 6.82
pH = 6.82

