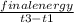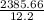Chemistry, 21.04.2020 08:47 sydneepollitt32
3. A student constructs a coffee cup calorimeter and places 50.0 mL of water into it. After a brief period of stabilization, the temperature of the water in calorimeter is determined to be 19.1 °C. To this is added 50.0 mL of water that was originally a temperature of 54.9 °C. A careful plot of the recorded temperature established T0 as 31.3 °C. What is the calorimeter constant (J/°C)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
3. A student constructs a coffee cup calorimeter and places 50.0 mL of water into it. After a brief...
Questions

Mathematics, 23.03.2020 22:37





Mathematics, 23.03.2020 22:37



Mathematics, 23.03.2020 22:37



Mathematics, 23.03.2020 22:37





Mathematics, 23.03.2020 22:37

History, 23.03.2020 22:37