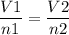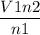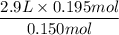QUESTION 5
A balloon has been filled to a volume of 2.90 L with 0.150 mol of helium gas. If 0....

QUESTION 5
A balloon has been filled to a volume of 2.90 L with 0.150 mol of helium gas. If 0.0450 mol of additional helium is added to the balloon while
the temperature and pressure are held constant, what is the new volume of the balloon?
Hint: Avogadro's law is V7/n1=V2/n2
A. 3.95 L
B. 3.77 L
C. 6.54 L
D. 5.45 L

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
Questions

Mathematics, 01.08.2019 19:30





Mathematics, 01.08.2019 19:30


History, 01.08.2019 19:30


History, 01.08.2019 19:30

Mathematics, 01.08.2019 19:30

Mathematics, 01.08.2019 19:30

Mathematics, 01.08.2019 19:30



Physics, 01.08.2019 19:30