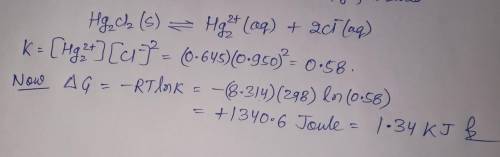Chemistry, 21.04.2020 04:51 micahwilkerson9495
A chemist fills a reaction vessel with 0.623g mercurous chloride(Hg2Cl2) solid, 0.645M mercury (I) (Hg2^2+)aqueous solution, and 0.905M chloride (Cl-) aqueous solution at a temperature of 25.0°C.
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction:
Hg2Cl2(s) ⇌ Hg2^2+ (aq) + 2Cl^- (aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
A chemist fills a reaction vessel with 0.623g mercurous chloride(Hg2Cl2) solid, 0.645M mercury (I) (...
Questions

Mathematics, 02.02.2021 18:40

Mathematics, 02.02.2021 18:40



Mathematics, 02.02.2021 18:40

Mathematics, 02.02.2021 18:40

English, 02.02.2021 18:40

Mathematics, 02.02.2021 18:40


Mathematics, 02.02.2021 18:40






Mathematics, 02.02.2021 18:40


Mathematics, 02.02.2021 18:40


Mathematics, 02.02.2021 18:40