Chemistry, 21.04.2020 01:50 chanel2371
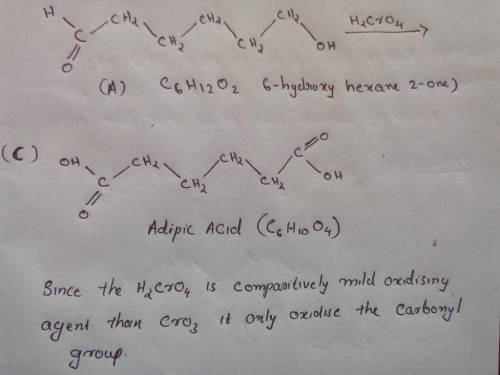
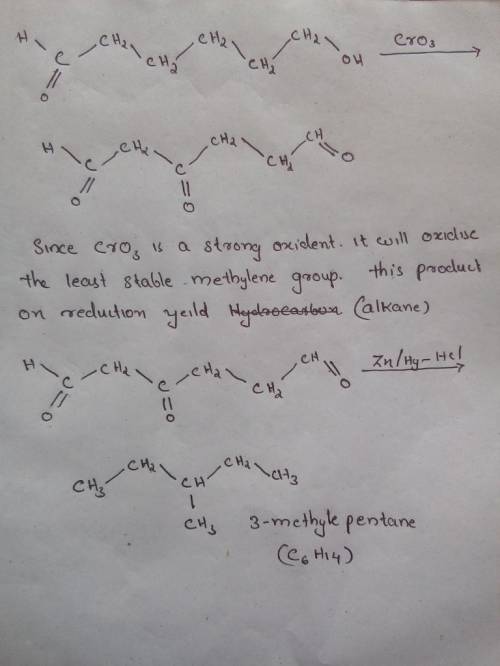
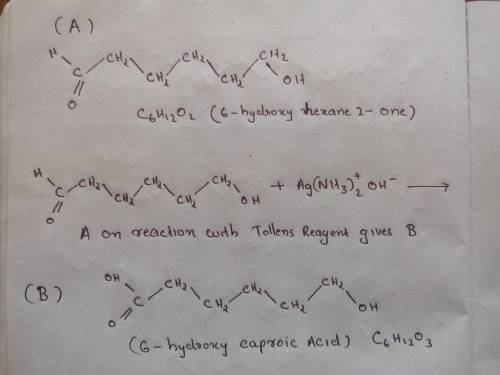
Compound B, C6H12O2, was found to be optically active, and it was oxidized to an optically active carboxylic acid A, by Ag (aka, Tollens Reagent). Oxidation of B by PCC gave an optically inactive compound X that reacted with Zn amalgam/HCl to give 3-methylpentane. With Na2Cr2O7/H2SO4, compound B was oxidized to an optically inactive dicarboxylic acid C, C6H10O4. Provide the structures of A, B, and C (ignore specific configuration of any stereocenters).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Compound B, C6H12O2, was found to be optically active, and it was oxidized to an optically active ca...
Questions

Mathematics, 25.10.2021 20:20

Social Studies, 25.10.2021 20:20


English, 25.10.2021 20:20

Biology, 25.10.2021 20:20

Mathematics, 25.10.2021 20:20

Mathematics, 25.10.2021 20:20





Mathematics, 25.10.2021 20:30


Mathematics, 25.10.2021 20:30

Law, 25.10.2021 20:30



SAT, 25.10.2021 20:30

Advanced Placement (AP), 25.10.2021 20:30

English, 25.10.2021 20:30

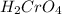 is comparatively mild oxidizing agent than the
is comparatively mild oxidizing agent than the 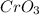 , it only oxidizes the carbon group
, it only oxidizes the carbon group