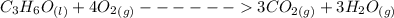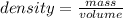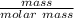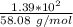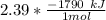Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover. c3h6o (l) + 4 o2 (g) à 3 co2 (g) + 3 h2o (g) ∆horxn = -1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions

History, 10.12.2020 18:20


Mathematics, 10.12.2020 18:20

Mathematics, 10.12.2020 18:20

Arts, 10.12.2020 18:20




Mathematics, 10.12.2020 18:20

Mathematics, 10.12.2020 18:20

Mathematics, 10.12.2020 18:20

Mathematics, 10.12.2020 18:20

Computers and Technology, 10.12.2020 18:20


Business, 10.12.2020 18:20

Spanish, 10.12.2020 18:20

Mathematics, 10.12.2020 18:20


Mathematics, 10.12.2020 18:20

Spanish, 10.12.2020 18:20