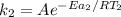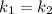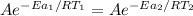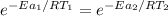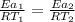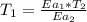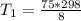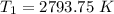Chemistry, 21.04.2020 00:11 hannah2757
Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the reaction equation 2h2o2(aq)⟶2h2o(l)+o2(g) the activation energy for this reaction is 75 kj·mol−1. the enzyme catalase, found in blood, lowers the activation energy to 8.0 kj·mol−1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25 °c?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the reaction e...
Questions


Geography, 30.07.2021 16:30

Mathematics, 30.07.2021 16:30

Social Studies, 30.07.2021 16:30



Mathematics, 30.07.2021 16:30

Mathematics, 30.07.2021 16:30

Computers and Technology, 30.07.2021 16:30

Computers and Technology, 30.07.2021 16:30

Social Studies, 30.07.2021 16:30

Biology, 30.07.2021 16:30

Mathematics, 30.07.2021 16:30

Mathematics, 30.07.2021 16:30

Mathematics, 30.07.2021 16:30



Mathematics, 30.07.2021 16:30


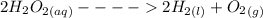
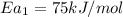
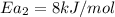
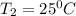 = (25+273)K = 298 K
= (25+273)K = 298 K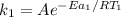 ----- equation (1)
----- equation (1)