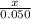Chemistry, 21.04.2020 00:04 nayelialvarez5950
Mg (s) + 2HCl (aq) → H2 (g) + MgCl2 (aq)
A:
Moles Mg: 0.050
Moles HCl: 0.050
Mass of Hydrogen gas and the limiting reactant

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
Mg (s) + 2HCl (aq) → H2 (g) + MgCl2 (aq)
A:
Moles Mg: 0.050
Moles HCl: 0.050
...
A:
Moles Mg: 0.050
Moles HCl: 0.050
...
Questions

Mathematics, 06.04.2021 22:30










Mathematics, 06.04.2021 22:30


Mathematics, 06.04.2021 22:30

Mathematics, 06.04.2021 22:30



Social Studies, 06.04.2021 22:30

Geography, 06.04.2021 22:30

Mathematics, 06.04.2021 22:30

Mathematics, 06.04.2021 22:30

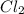 (aq)
(aq)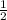 =
=