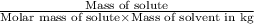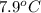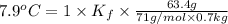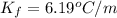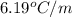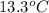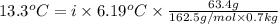Chemistry, 20.04.2020 20:50 loganrose50
When 63.4 g of glycine (C2HNO2 are dissolved in 700. g of a certain mystery liquid X, the freezing point of the solution is 7.9 °C lower than the freezing point of pure X. On the other hand, when 63.4 g of iron(III) chloride are dissolved in the same mass of X, the freezing point of the solution is 13.3 °C lower than the freezing point of pure X Calculate the van't Hoff factor for iron(III) chloride in X. Be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits. x 10

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
When 63.4 g of glycine (C2HNO2 are dissolved in 700. g of a certain mystery liquid X, the freezing p...
Questions

English, 20.05.2021 01:20

Physics, 20.05.2021 01:20

Business, 20.05.2021 01:20


Spanish, 20.05.2021 01:20

Chemistry, 20.05.2021 01:30

Mathematics, 20.05.2021 01:30



English, 20.05.2021 01:30



Business, 20.05.2021 01:30

Social Studies, 20.05.2021 01:30

Mathematics, 20.05.2021 01:30


English, 20.05.2021 01:30


Chemistry, 20.05.2021 01:30

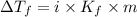
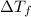 =depression in freezing point =
=depression in freezing point = 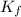 = freezing point constant
= freezing point constant