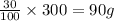Chemistry, 20.04.2020 20:47 Alienhead6187
An ionic compound has a solubility of 30 g per 100 mL of water at room temperature. A solution containing 70 g of the compound in 300 mL of water at the same temperature is:
A. unsaturated.
B. saturated.
C. a suspension.
D. supersaturated.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


You know the right answer?
An ionic compound has a solubility of 30 g per 100 mL of water at room temperature. A solution conta...
Questions




History, 04.01.2020 13:31

Physics, 04.01.2020 13:31

Mathematics, 04.01.2020 13:31

Chemistry, 04.01.2020 13:31

Mathematics, 04.01.2020 13:31

Biology, 04.01.2020 13:31

Spanish, 04.01.2020 13:31

Mathematics, 04.01.2020 13:31

Chemistry, 04.01.2020 13:31


Mathematics, 04.01.2020 13:31


Biology, 04.01.2020 13:31

Mathematics, 04.01.2020 13:31


Biology, 04.01.2020 13:31

Geography, 04.01.2020 13:31