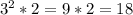Chemistry, 20.04.2020 19:53 angiezavala61
Please consider the following gas phase reaction and its experimentally determined rate law expression. If the concentration of A is tripled and the concentration of B is doubled, the reaction rate would be increased by a factor of:.
A + B → C rate = k[A]^2 [B]
A) 6
B) 9
C) 12
D) 18
E) 36

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Please consider the following gas phase reaction and its experimentally determined rate law expressi...
Questions




Mathematics, 16.03.2022 18:40








Computers and Technology, 16.03.2022 18:40

Biology, 16.03.2022 18:40





SAT, 16.03.2022 18:50

Geography, 16.03.2022 18:50

![r=k[A]^2[B]](/tpl/images/0612/4716/2d753.png)
![r=k[3*A]^2[2*B]](/tpl/images/0612/4716/87b43.png)