
Chemistry, 20.04.2020 04:58 alexmoy45p8yd7v
50.g of NaNO3 was dissolved in 1250 mL of water. what is the molality of the solution? [ Molar mass of NaNO3 = 85 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1
You know the right answer?
50.g of NaNO3 was dissolved in 1250 mL of water. what is the molality of the solution? [ Molar mass...
Questions


English, 12.05.2021 23:40


English, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40



Mathematics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40

English, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40


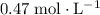 (note that
(note that 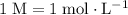 .)
.)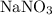 solution in water,
solution in water, 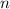 be the number of moles of the solute in the whole solution. Let
be the number of moles of the solute in the whole solution. Let 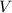 represent the volume of that solution. The formula for the molarity
represent the volume of that solution. The formula for the molarity  of that solution is:
of that solution is: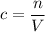 .
.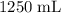 . That's
. That's 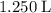 in standard units. What needs to be found is
in standard units. What needs to be found is 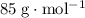 means that the mass of one mole of
means that the mass of one mole of 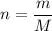 .
.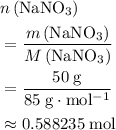 .
.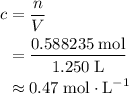 .
.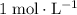 (one mole per liter solution) is the same as
(one mole per liter solution) is the same as 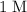 .
. 

