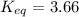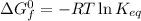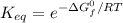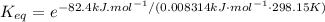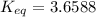Calculate the value of the equilibrium constant (K) at 25 °C for the reaction
2 NOBr(g) = N2(g)...

Chemistry, 20.04.2020 03:26 officialkk
Calculate the value of the equilibrium constant (K) at 25 °C for the reaction
2 NOBr(g) = N2(g) + O2(g) + Br2()
given that the standard free energy of formation (AG°f) of NOBr(g) is 82.4 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
Questions


Health, 19.07.2019 12:00

Advanced Placement (AP), 19.07.2019 12:00




Mathematics, 19.07.2019 12:00

Mathematics, 19.07.2019 12:00






Biology, 19.07.2019 12:00

Mathematics, 19.07.2019 12:00



Biology, 19.07.2019 12:00

History, 19.07.2019 12:00